If you go to Mars, you’ll be stuck there unless scientists figure out a way to make the rocket fuel to bring you back. (And mitigate myriad other risks.)
Think how much rocket fuel it would take to go 34 million miles and then double it to come back!
There are plenty of ideas, but none of them have been tried outside a lab.
One such theory is converting carbon dioxide, of which there is plenty on Mars, into methane. How would you do this?
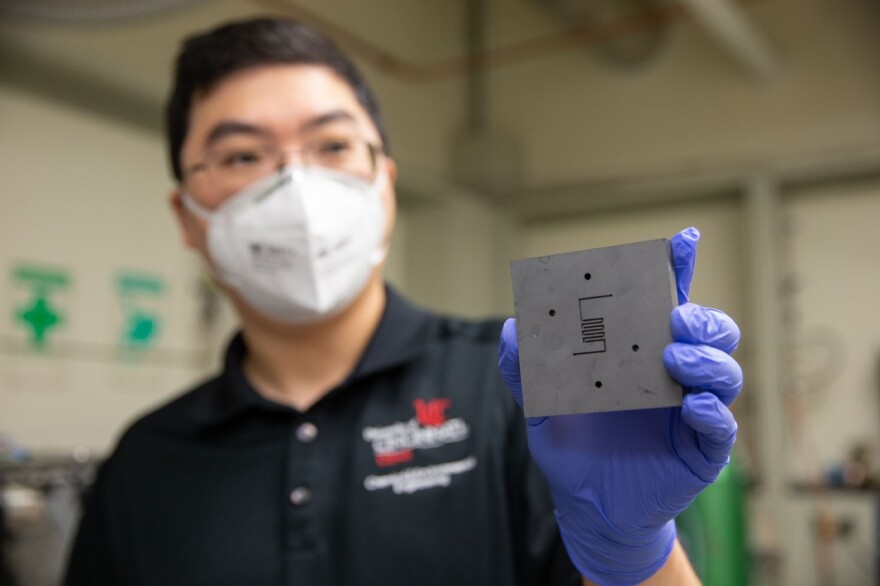
UC College of Engineering and Applied Science Assistant Professor Jingjie Wu says you could easily pump carbon dioxide, water and a carbon catalyst through a reactor and produce methane for a rocket.
"It’s like a gas station on Mars,” he says.
The astronauts on the International Space Station use a Sabatier reactor to clean up the air they breathe and to make rocket fuel that keeps the ISS in orbit.
The University of Cincinnati collaborated with Rice University in Houston, Shanghai University and East China University of Science and Technology to test this method and publish an article in the journal Nature Communications.
Here’s How Easy It Could Be
“There is a lot of carbon dioxide - 90 to 95% - so we can convert carbon dioxide to methane,” Wu explains. The catalyst would be a very small bit of carbon on the nanoscale.
And the time it would take to put the ingredients in would be the time it takes to make the fuel. “You can scale up the reactor as big as you need," he says. "That’s not a problem.”
Other ideas:
- NASA wants to produce a renewable liquid that could be produced and burned on Mars.
- Other researchers have proposed using zinc as a catalyst to make methane from carbon dioxide and ice.
What Does This Mean On Earth?
This process could help minimize climate change and produce fuel as a byproduct.
“The process is 100 times more productive than it was just 10 years ago," Wu says. "So, you can imagine that progress will come faster and faster. In the next 10 years we’ll have a lot of startup companies to commercialize this technique.”




